Leadgene Biomedical, Inc.

A New Era in Renal Diagnostics
Leadgene Biomedical has developed a groundbreaking in vitro diagnostic solution for the precise, efficient, and cost-effective assessment of kidney function.
The Leadgene® Indoxyl Sulfate (IS) ELISA Kit is the world’s first ELISA assay approved by CE-IVD, VN MAC, HAS, and TFDA for the detection of indoxyl sulfate (IS)—a novel biomarker for chronic kidney disease (CKD). It provides a powerful, accessible alternative to complex conventional methods, offering comparable accuracy with improved usability.
Designed for high specificity and sensitivity, the kit demonstrates strong correlation with clinical indicators such as eGFR and serum creatinine, and has been validated in multiple peer-reviewed studies for its clinical relevance in CKD progression monitoring and dialysis patient management.
With a user-friendly protocol, rapid results, and no need for costly instrumentation, this IVD enables hospitals, diagnostic labs, and research institutions to monitor IS levels reliably and affordably.
More than just a diagnostic test, the Leadgene® IS ELISA Kit is a strategic tool driving the future of precision nephrology.
Products

LGM-18002 Leadgene Indoxyl Sulfate (IS) ELISA Kit
This innovative certified IVD enables accurate, cost-effective renal function assessment by detecting a novel CKD biomarker—with high specificity, validated usability, and fast results.
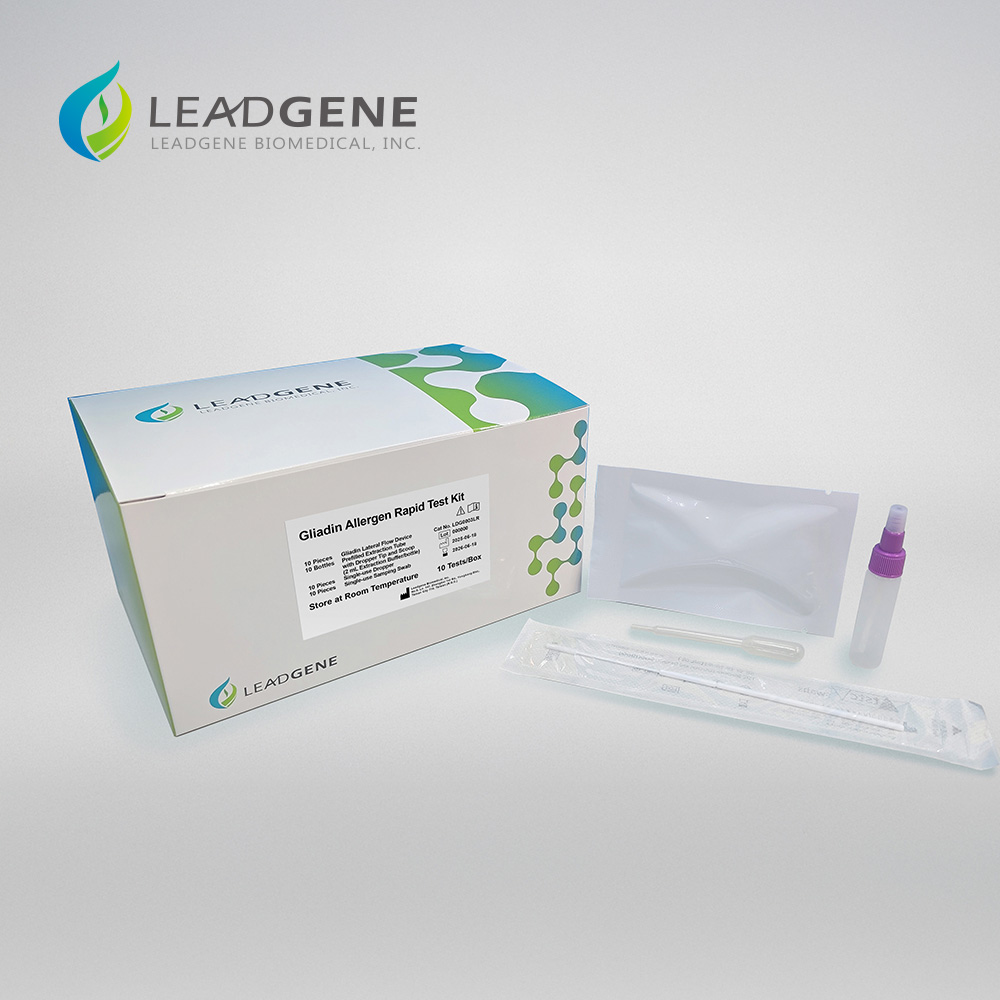
LDG0003LR Gliadin Allergen Rapid Test Kit
Leadgene® Gliadin Allergen Rapid Test Kit offers fast and reliable on-site detection of gliadin, a gluten protein commonly found in wheat, barley, and rye. Ideal for food manufacturers and safety inspectors, this user-friendly lateral flow assay delivers clear results within 10 minutes—ensuring gluten-free compliance and protecting consumers with gluten sensitivities or celiac disease.

Disease Detection Platform
Leadgene Biomedical has developed a series of rapid test kits for detecting common infectious diseases such as hMPV, Nipah virus, and Mycoplasma hyopneumoniae in swine. Built on advanced lateral flow immunoassay technology, the Leadgene Disease Detection Platform offers simple operation, rapid results, and clear interpretation. These kits are well-suited for clinical research, epidemiological surveillance, and laboratory-based screening.

Leadgene Biomedical, Inc.
Angela Tai Tel: +886 6 2536677
No.9, Ln. 147, Zhengbei 1st Rd., Yongkang Dist., Tainan City 71042, Taiwan
